I wish to all a happy 2012! :)
This blog intends to display concepts, informations, musics, videos, games, cartoons, curiosities about biochemical issues. Because Biochemistry does not have to be incomprehensible...
Pages
▼
Saturday, December 31, 2011
Thursday, December 29, 2011
Tuesday, December 27, 2011
Sunday, December 25, 2011
Music about the thermodynamics of metabolism
The song Yesterday, from The Beatles, was used to create a music about the thermodynamics of the cellular metabolism. Here it is the final result, directly drom Dr. Ahern's imagination (www.davincipress.com/
http://www.mediafire.com/?2fqq9en6ujrktbn
The Cell's Lament
Instructor sings
Woe is me
My substrates are losing entropy
Causing gains in Gibbs free energy
Oh I can't lose no en - tro- py
Re-a-lly
I could use a source of enthalpy
To combat the rise in Delta G
Oh I believe in enthalpy
Everyone sings
I crave en-er-gy
Don't you see?
It's getting worse
My re-actions all
Soon will stall
And then rever-r-r-se
Instructor sings
ATP
It's the metabolic currency
Guess I'll spend a bit judiciously
To help reduce the Delta G
Help reduce the Delta G
http://www.mediafire.com/?2fqq9en6ujrktbn
The Cell's Lament
Instructor sings
Woe is me
My substrates are losing entropy
Causing gains in Gibbs free energy
Oh I can't lose no en - tro- py
Re-a-lly
I could use a source of enthalpy
To combat the rise in Delta G
Oh I believe in enthalpy
Everyone sings
I crave en-er-gy
Don't you see?
It's getting worse
My re-actions all
Soon will stall
And then rever-r-r-se
Instructor sings
ATP
It's the metabolic currency
Guess I'll spend a bit judiciously
To help reduce the Delta G
Help reduce the Delta G
Saturday, December 24, 2011
Merry Christmas!
I wish to all visitors of World of Biochemistry, and particularly to its followers, a very Merry Christmas, fukk of peace and joy! :)
Thursday, December 22, 2011
Tuesday, December 20, 2011
Sunday, December 18, 2011
Friday, December 16, 2011
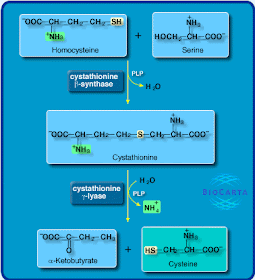
Thiol group
 The thiol group, or sulphydryl group or mercaptan group, is the functional group –SH. The prefix "thio" in this, and other functional groups, means that it has a sulfur atom instead of an oxygen (for example, there are also the thioester and thioether groups...). In this case, the ending suffix "ol", tells us that indeed this group is a group derived from an alcohol. That is, instead of the hydroxyl group –OH, we have sulfur in the place of the oxygen. One very important difference with respect to hydroxyl groups is related to the fact that the thiol groups present a weak acid behavior.
The thiol group, or sulphydryl group or mercaptan group, is the functional group –SH. The prefix "thio" in this, and other functional groups, means that it has a sulfur atom instead of an oxygen (for example, there are also the thioester and thioether groups...). In this case, the ending suffix "ol", tells us that indeed this group is a group derived from an alcohol. That is, instead of the hydroxyl group –OH, we have sulfur in the place of the oxygen. One very important difference with respect to hydroxyl groups is related to the fact that the thiol groups present a weak acid behavior.Since it has sulfur in its composition, the presence of thiol groups often confers to molecules an unpleasant odor.
Tuesday, December 13, 2011
Game about acids and bases (3)
Here it goes the link to a game about acids and bases.
http://pbskids.org/zoom/games/kitchenchemistry/virtual-start.html
http://pbskids.org/zoom/games/kitchenchemistry/virtual-start.html
Sunday, December 11, 2011
Famous sentence (6)
Age is an issue of mind over matter. If you don’t mind, it doesn’t matter." - Mark Twain
Saturday, December 10, 2011
Friday, December 9, 2011
Thursday, December 8, 2011
Music about biochemistry (2)
Here it goes another song about biochemistry made by Dr. Ahern (www.davincipress.com/
http://www.mediafire.com/?2zsrhqcgs2gkg2u
Brain Farts Just Happen In My Head
Brain farts just happen in my head
I think it might be due to something Kevin said
Bi-o-che-mis-try
Gets brain farts a poppin' in my head and they're poppin'
So I just wiped out the teardrops from my eyes
And told my brain it had to do some men-tal exer-cise
Burn some ATP
So brain farts can stop inside my head they'll be stoppin'
'Cause there's one thing I've learned
When energy increases it sure pleases
My mental state - I'm doing great as tension eases
Now brain farts don't happen in my head
So I'm sure the final will be easier instead
Cyclic AMP
Stops brain farts from poppin' in my head they're not poppin'
Thanks to caffeine
Nothin's worryin' me
Wednesday, December 7, 2011
Animation about a proton pump
Here it is the link to download an animation about the active transport of H+.
http://www.mediafire.com/?p4jouhjecojumdn
http://www.mediafire.com/?p4jouhjecojumdn
Tuesday, December 6, 2011
Monday, December 5, 2011
Sunday, December 4, 2011
Ether group
The ether functional group is characterized by the presence of an oxygen atom shared by two carbons (can be alkyl or aryl groups). It is therefore a functional group that appears in an internal position within the molecules. Its general formula is C1-O-C2.

The C-O-C bond angle has about 104.5 °, with a bonding distance of 140pm. The ether group is a polar functional group, because the COC bond angle is not 180°, which impairs the molecule dipoles to cancel each other. Although it cannot function as an H donor in hydrogen bonds, it can function as an acceptor of them.
In biochemistry, the ether groups are present, for example, in the O-glycosidic bonds.
Saturday, December 3, 2011
Scientific jokes (9)
Did you hear about the biologist who had twins?
She baptized one and kept the other as a control.
She baptized one and kept the other as a control.
Friday, December 2, 2011
Thursday, December 1, 2011
Game about acids and bases (2)
Here it is a link to a game about acids and bases, in a more physiological perspective.
http://www.funtrivia.com/playquiz/quiz2398821b771b8.html
http://www.funtrivia.com/playquiz/quiz2398821b771b8.html
Wednesday, November 30, 2011
Music about biochemistry
Here it goes one more Christmas song (Oh Christmas tree) that was used to make a different song, this time about Biochemistry. Thanks Dr. Ahern (www.davincipress.com/
Biochemistry/Biochemistry
Biochemistry Biochemistry
I wish that I were wiser
I feel I'm in way o'er my head
I need a new advisor
My courses really shouldn't be
Such metabolic misery
Biochemistry Biochemistry
I wish that I were wiser
Biochemistry Biochemistry
Reactions make me shiver
They're in my heart and in my lungs
They're even in my liver
I promise I would not complain
If I could store them in my brain
Biochemistry Biochemistry
I wish that I were wiser
Biochemistry Biochemistry
I'm truly in a panic
Your mechanisms murder me
I should have learned organic
For all I have to memorize
I ought to win the Nobel Prize.
Biochemistry Biochemistry
I wish that I were wiser
Biochemistry/Biochemistry
Biochemistry Biochemistry
I wish that I were wiser
I feel I'm in way o'er my head
I need a new advisor
My courses really shouldn't be
Such metabolic misery
Biochemistry Biochemistry
I wish that I were wiser
Biochemistry Biochemistry
Reactions make me shiver
They're in my heart and in my lungs
They're even in my liver
I promise I would not complain
If I could store them in my brain
Biochemistry Biochemistry
I wish that I were wiser
Biochemistry Biochemistry
I'm truly in a panic
Your mechanisms murder me
I should have learned organic
For all I have to memorize
I ought to win the Nobel Prize.
Biochemistry Biochemistry
I wish that I were wiser
Tuesday, November 29, 2011
Monday, November 28, 2011
Animation about crossingover
Here it is a link to download an animation about crossingover.
http://www.mediafire.com/?3fbcjo1l1c4kjw6
http://www.mediafire.com/?3fbcjo1l1c4kjw6
Sunday, November 27, 2011
Saturday, November 26, 2011
Friday, November 25, 2011
Amide group
 The amide group is originated when a carboxyl group reacts with an amine group. It is characterized by the presence of a carbon bounded to an oxygen through a double bond and to a nitrogen via a single bond.
The amide group is originated when a carboxyl group reacts with an amine group. It is characterized by the presence of a carbon bounded to an oxygen through a double bond and to a nitrogen via a single bond.
The amide group can be located at the end of a molecule, in which case the nitrogen is connected to two hydrogens, or may be in an internal position, causing the nitrogen to be connected to a hydrogen and a second carbon, or two different carbons.
Despite having a nitrogen atom, the amide group does not have a significant alkaline behavior.
In biochemistry the amide group appears in a prominent position because it is the functional group that exists in the peptide bonds.
In biochemistry the amide group appears in a prominent position because it is the functional group that exists in the peptide bonds.
The amide groups can be identified by infrared spectroscopy (it shows a VCO band at about 1650 cm-1).
Thursday, November 24, 2011
Game to kill aminoacids
This is my favourite biochemical game. You can download it with the link:
http://www.mediafire.com/?vf10cvmo6nsray9
http://www.mediafire.com/?vf10cvmo6nsray9
Wednesday, November 23, 2011
Tuesday, November 22, 2011
Monday, November 21, 2011
Sunday, November 20, 2011
Saturday, November 19, 2011
Music about DNA
Is there anyone who does not know the music YMCA? Here it is a version of it about DNA, made by Dr. Ahern (www.davincipress.com/
http://www.mediafire.com/?v0lk6tqayxgjpcb
http://www.mediafire.com/?v0lk6tqayxgjpcb
B-DNA
Phosphates
Are in nucleotides
I say phosphates
Cover bases inside
I say phosphates
Span the 5 and 3 primes
There's no need - to - be - all - mixed - up
Bases
Carry info you see
I say bases
Are all complement'ry
I say bases
Like A,T,G and C
They have got - to - be – all - paired - up
It's fun to play with some
B-DNA
It's got a boatload of
G-C-T-A
It's got everything
A polymerase needs
When you melt all the A's and T's
It's fun to play with some
B-DNA
It's got a boatload of
G-C-T-A
You can make RNAs
With a po-ly-mer-ase
Just by pairing up U's with A's
Proteins
Full of amino A's
I say proteins
Come from mRNAs
I say proteins
Require tRNAs
There is more – you - need - to – trans-late
Codons
Like our friend U-A-C
I say codons
Come in clusters of three
I say codons
Have one base wobble –ee
Now you can - go - forth - and - tran-slate
It's fun to play with some
B-DNA
It's got a boatload of
G-C-T-A
All those hydrogen Bs
And right-hand he-li-ces
Anti-par-a-llel fives and threes
It's fun to play with some
B-DNA
It's got a boatload of
G-C-T-A
All those hydrogen Bs
And right-hand he-li-ces
Anti-par-a-llel fives and threes
It's fun to play with some
B-DNA
It's got a boatload of
G-C-T-A
All those hydrogen Bs
And right-hand he-li-ces
Anti-par-a-llel fives and threes
Friday, November 18, 2011
Thursday, November 17, 2011
Wednesday, November 16, 2011
Ester group
 The ester group is characterized by being an internal functional group, that is, it does not appear in the ends of the molecules. It presents two oxygen atoms bound to the same carbon atom, one through a double bound and the other one through a single one (R1-COO-R2). It is a neutral polar group, that is, it does not possess acid or basic properties. It can establish hydrogen bounds, acting as a H acceptor.
The ester group is characterized by being an internal functional group, that is, it does not appear in the ends of the molecules. It presents two oxygen atoms bound to the same carbon atom, one through a double bound and the other one through a single one (R1-COO-R2). It is a neutral polar group, that is, it does not possess acid or basic properties. It can establish hydrogen bounds, acting as a H acceptor.
It is formed by the reaction of a carboxyl and a hydroxyl functional groups. Putting it in a simple way, it is the result of the substitution of the hydrogen atom of a carboxyl group by an organic portion.
It can be identified by infrared sepctroscopy (it presents a band between 1730-1750 cm-1).
Sunday, November 13, 2011
Animation about southern blot
Here it goes the link to download an animation about southern blot.
http://www.mediafire.com/?nrloo2h3q240cy7
http://www.mediafire.com/?nrloo2h3q240cy7
Saturday, November 12, 2011
Friday, November 11, 2011
Thursday, November 10, 2011
Wednesday, November 9, 2011
Music about fermentation (2)
This is one more Christmas song (Rudolph the Red-Nosed Reindeer) that turned out into a song about biochemistry, this time about fermentation. Thank you Dr. Ahern (www.davincipress.com/
http://www.mediafire.com/?5afx4u8ed6uk80l
The Alcohol Song
Substrate phosphorylation’s
How they make their ATP
With no electron takers
They need an alternative
Isn’t fermentation great?
For reducing pyruvate!
Ask yourself when you’re drinking
“Where do we get alcohol?”
http://www.mediafire.com/?5afx4u8ed6uk80l
The Alcohol Song
Cells go through fermentation
When they’re out of NADSubstrate phosphorylation’s
How they make their ATP
Cells are efficient makers ( . . .of)
Energy on which to liveWith no electron takers
They need an alternative
Oh glycolysis would stop
Without NADIsn’t fermentation great?
For reducing pyruvate!
And if you might be thinking
“Man this isn’t cool at all”Ask yourself when you’re drinking
“Where do we get alcohol?”
Tuesday, November 8, 2011
Monday, November 7, 2011
Sunday, November 6, 2011
Saturday, November 5, 2011
Amino group
The amine group is characterized by the presence of a nitrogen atom with a pair of non-bonding electrons. It is derived from ammonia, where one or more hydrogens have been replaced by other atoms.
The amines can be divided into primary, secondary and tertiary. The primaries are those in which only one of three hydrogen atoms of ammonia was replaced by an alkyl chain. The secondary amines have two alkyl chains and the tertiary have three. The secondary and tertiary amines may be presented in a linear or cyclic form.
The amines may be designated in different ways. Typically, it is used the prefix amino-("aminoacid", for example) or the suffix-amine ("glucosamine," for example). When there is a substitution in the amino group, it is named starting with the prefix N-.
The amines can establish hydrogen bonds, since they have one H atom attached to a very electronegative atom (N in this case).
The amines act as weak bases, as they can capture a H+, due to the existence of a pair of non-bonding electrons in the nitrogen atom. They are the most relevant alkaline functional group in biochemistry.
The amines can establish hydrogen bonds, since they have one H atom attached to a very electronegative atom (N in this case).
The amines act as weak bases, as they can capture a H+, due to the existence of a pair of non-bonding electrons in the nitrogen atom. They are the most relevant alkaline functional group in biochemistry.
Friday, November 4, 2011
Thursday, November 3, 2011
Music about hemoglobin
Christmas is becoming near and a well known Christmas song (Santa Claus is Coming to Town) was adapted by Dr. Ahern (www.davincipress.com/
Hemoglobin's Moving Around
Oh isn't it great
What proteins can do
Especially ones that bind to O
Hemoglobin's moving around
Inside of the lungs
It picks up the baitAnd changes itself from T to R state
Hemoglobin's moving around
The proto-porphyrin system
Its iron makes such a sceneArising when an O binds
Pulling up on histidine
The binding occurs
Cooperatively
Thanks to changes qua-ter-nar-y
Hemoglobin's moving around
It exits the lungs
Engorged with O
In search of a working body tissue
Hemoglobin's moving aroundThe proton concentration
Is high and has a role
Between the alpha betas
It finds imidazole
To empty their loads
The globins decree"We need to bind 2,3BPG"
Hemoglobin's moving around
The stage is thus set
For grabbing a few
Cellular dumps of CO
Hemoglobin's moving around
And then inside the lungs it
Discovers ox-y-gen
And dumps the CO off
To start all o'er agin
So see how this works
You better expect
To have to describe the Bohr effect
Hemoglobin's moving around
Wednesday, November 2, 2011
Game about functional groups (3)
One more quiz about functional groups... :)
http://www.funtrivia.com/playquiz/quiz30262422a5408.html
http://www.funtrivia.com/playquiz/quiz30262422a5408.html
Tuesday, November 1, 2011
Famous sentence (3)
"The essence of life is statistical improbability on a colossal scale.” - Richard Dawkins.
Monday, October 31, 2011
Sunday, October 30, 2011
Saturday, October 29, 2011
Friday, October 28, 2011
Thursday, October 27, 2011
Wednesday, October 26, 2011
Carboxyl group
 The carboxyl group is composed of two oxygen atoms bound to a carbon, one by a double bond and the other through a single bond. The latter is also covalently bonded to a hydrogen atom (R-COOH). Put simply, it can be seen as a functional group comprising a carbonyl group and a hydroxyl group.
The carboxyl group is composed of two oxygen atoms bound to a carbon, one by a double bond and the other through a single bond. The latter is also covalently bonded to a hydrogen atom (R-COOH). Put simply, it can be seen as a functional group comprising a carbonyl group and a hydroxyl group.
The carboxyl group is the main acidic group in organic chemistry, and therefore can lose an H+, resulting in an anionic carboxylate group (R-COO-). Carboxylate ions are stabilized by electronic resonance, which allows the carboxyl group to present a significant acidic behavior. Carboxyl groups are highly polar and can act as H donors and acceptors in hydrogen bonds.
In biochemistry, the carboxyl groups are sometimes removed from biomolecules in the so-called decarboxylation reactions (they are released in the form of CO2).
The presence of carboxyl groups in the molecules can be detected by infrared spectroscopy, showing a characteristic vibration between 1680 and 1725 cm-1. Alternatively, it can be identified by nuclear magnetic resonance, appearing in the region of 10-13 ppm.
The presence of carboxyl groups in the molecules can be detected by infrared spectroscopy, showing a characteristic vibration between 1680 and 1725 cm-1. Alternatively, it can be identified by nuclear magnetic resonance, appearing in the region of 10-13 ppm.
Tuesday, October 25, 2011
Monday, October 24, 2011
Sunday, October 23, 2011
Animation about a weak acid
Here it is a link to download a very simple animation about what happens to a weak acid in solution.
http://www.mediafire.com/?lyrqabzcs6aywwu
Saturday, October 22, 2011
Game about functional groups (2)
Another game about functional groups... :)
http://firstyear.chem.usyd.edu.au/games/flashcard.shtml?select=functional_groups
http://firstyear.chem.usyd.edu.au/games/flashcard.shtml?select=functional_groups
Friday, October 21, 2011
Thursday, October 20, 2011
Biochemical curiosities (5)
To prevent odors in the refrigerator it can be used an open box of baking soda. It completely absorbs all the odors from stored food.
Wednesday, October 19, 2011
Tuesday, October 18, 2011
Monday, October 17, 2011
Hydroxyl group
 The hydroxyl group is a functional group characterized by the presence of an oxygen atom covalently bonded to a hydrogen atom (R-OH). It is also called alcohol group, and the molecules that contain it have in their names the suffix "ol". In biochemistry, this group is particularly important because it can act both as donor and acceptor of H in hydrogen bonds. Therefore, their presence in biomolecules increases their water solubility and hydrophilic character.
The hydroxyl group is a functional group characterized by the presence of an oxygen atom covalently bonded to a hydrogen atom (R-OH). It is also called alcohol group, and the molecules that contain it have in their names the suffix "ol". In biochemistry, this group is particularly important because it can act both as donor and acceptor of H in hydrogen bonds. Therefore, their presence in biomolecules increases their water solubility and hydrophilic character. This is a non-charged polar group. However, in some biochemical contexts it can function as a weak acid, losing one H+. This behavior is particularly important in the case of phenolic hydroxyl groups, i.e., in the case of hydroxyl groups bounded to benzene rings. As the main example of this situation, we have the amino acid tyrosine, which in some proteins may have an acidic behaviour.
This is a non-charged polar group. However, in some biochemical contexts it can function as a weak acid, losing one H+. This behavior is particularly important in the case of phenolic hydroxyl groups, i.e., in the case of hydroxyl groups bounded to benzene rings. As the main example of this situation, we have the amino acid tyrosine, which in some proteins may have an acidic behaviour.Sunday, October 16, 2011
Music about protein translation (2)
The music Maria from West Side Story has inspired Dr. Ahern (www.davincipress.com/
Translation
Translation
The most intricate thing I ever saw
From five prime to three prime, translation, translation
The final step that we know about the central dog-ma
Amino, carboxyl, translation, translation. . . .
Translation, translation, translation . .
Translation!
I just learned the steps of translation
And all the things they say
About tRNA
Are true
Translation!
To form peptide bonds in translation
The ribosomal cleft
Must bind to an E-F
tee-you!
Translation!
A-U-G binds the f-met's cargo
16S lines up Shine and Dalgarno
Translation
I'll never stop needing translation
The most intricate thing I ever saw
Translationnnnnnnnnnnnnnnnnn