This blog intends to display concepts, informations, musics, videos, games, cartoons, curiosities about biochemical issues. Because Biochemistry does not have to be incomprehensible...
Pages
- Home
- Functional groups
- Non-covalent interactions
- Isomers
- Carbohydrates
- Proteins
- Digestion of biomolecules
- Glycolysis and fates of pyruvate
- Krebs cycle
- Cellular respiration
- Glycogen metabolism and gluconeogenesis
- Pentose phosphate pathway
- Fatty acids metabolism
- Cholesterol metabolism
- Lipoproteins
- Aminoacids metabolism
Thursday, January 22, 2015
Friday, January 16, 2015
Tuesday, January 13, 2015
No comments...
It is said that "one image worths more than 1000 words", when I look to this photo I cannot agree more...
Thank you for another sugestion Filipe.
.
Thank you for another sugestion Filipe.
.
Friday, January 9, 2015
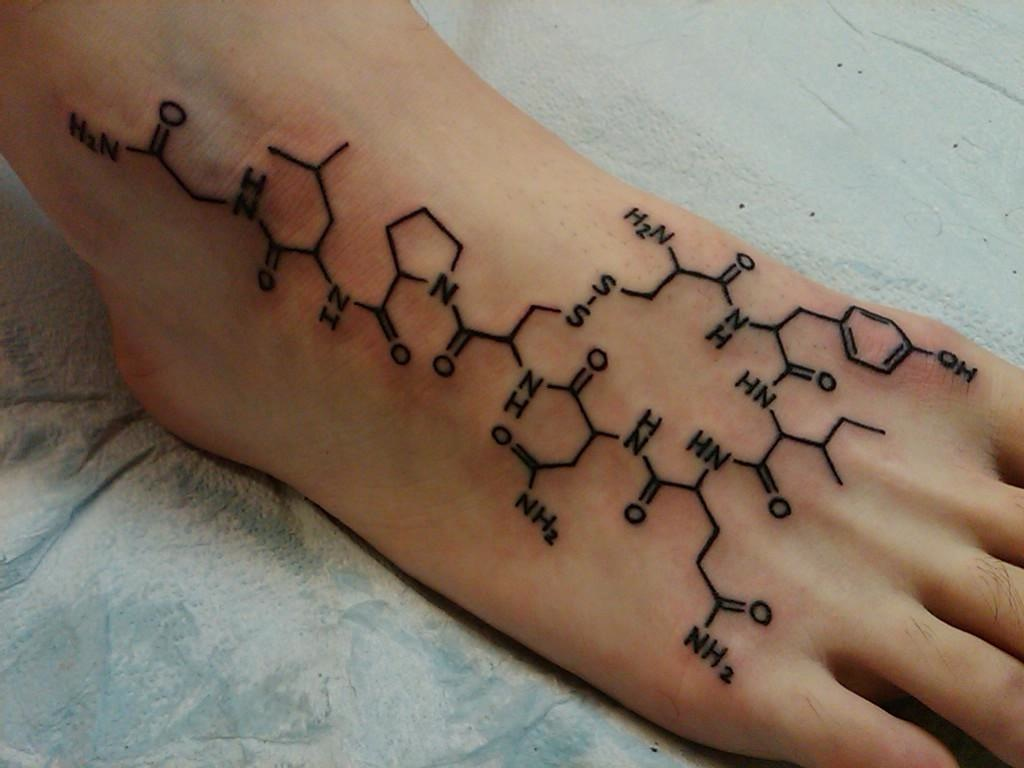
Tattoo - oxytocin
Here are some examples of tattos about the "love hormone" - oxytocin. Love is complex and so it is the chemical structure of oxytocin! :)
Thank you for the input Filipe!
.
Thank you for the input Filipe!
.
Tuesday, January 6, 2015
Friday, January 2, 2015
Peptide bond
 The peptide
bond is a covalent bond established between two amino acids. But it is not a whatsoever
bond... it is a bond that involves the a-amine group of an amino acid and an a-carboxyl group of another amino
acid. The term peptide bond is sometimes used generically, when any amino group
of one amino acid reacts with a (any!) carboxyl group of another amino acid,
and this general use is shrouded in some controversy. In fact, some consider
that only when the functional groups involved in the bond are linked to an a carbon, the bond should be called
peptide, whereas when the functional groups are in different locations in the
corresponding amino acids, the bond should no longer receive this name. I
personally also agree that only in the first case it is a peptide bond.
The peptide
bond is a covalent bond established between two amino acids. But it is not a whatsoever
bond... it is a bond that involves the a-amine group of an amino acid and an a-carboxyl group of another amino
acid. The term peptide bond is sometimes used generically, when any amino group
of one amino acid reacts with a (any!) carboxyl group of another amino acid,
and this general use is shrouded in some controversy. In fact, some consider
that only when the functional groups involved in the bond are linked to an a carbon, the bond should be called
peptide, whereas when the functional groups are in different locations in the
corresponding amino acids, the bond should no longer receive this name. I
personally also agree that only in the first case it is a peptide bond. The
formation of a peptide bond is an example of a condensation reaction, which
releases a water molecule. Therefore, one of the amino acids involved in the
reaction will release O and H and the other will release H. Thus, since in the
final product, each of the two amino acids will not have exactly the same
composition which had initially, they should be called amino acid residues. The
peptide bond formation requires energy in the form of ATP hydrolysis, and that
is why protein synthesis is expensive from an energy point of view. The reverse
reaction (cleavage of the peptide bond) is a hydrolysis reaction and releases
energy.
The
formation of a peptide bond is an example of a condensation reaction, which
releases a water molecule. Therefore, one of the amino acids involved in the
reaction will release O and H and the other will release H. Thus, since in the
final product, each of the two amino acids will not have exactly the same
composition which had initially, they should be called amino acid residues. The
peptide bond formation requires energy in the form of ATP hydrolysis, and that
is why protein synthesis is expensive from an energy point of view. The reverse
reaction (cleavage of the peptide bond) is a hydrolysis reaction and releases
energy.
The peptide
bond is an amide bond (not amine as often incorrectly named!). From a
structural point of view, it is a planar bond, that means, all atoms are located
in the same plane.
Indeed, the bond is not a single bond, but it is a partially
double bond, since electronic delocalization exists between the nitrogen and
the oxygen. As a result, the peptide bonds are confer local structural rigidity
in the molecules that have them since they are not single bonds, and thus do
not suffer rotation. Therefore, they do not allow conformational changes in that
location. As happens with the double bonds, peptide bonds can exist in two
different isomeric forms: cis and trans.
Subscribe to:
Posts (Atom)